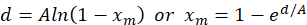Part 1. General Considerations
Currently, there are several different thermodynamic hydrate inhibitors that one has available to choose from. One could choose to use an oxygenate, such as methanol, or a glycol, such as such as mono-ethylene glycol (MEG) or diethylene glycol (DEG). But what determines the inhibitors hydrate suppression performance, and which one should I choose for my inhibition needs? The primary considerations for an application include:
- Hydrate Suppression Effectiveness
- Inhibitor Regeneration Requirements
- Inhibitor Losses / Product Contamination
- Inhibitor Cost
Hydrate Suppression Effectiveness
The lower the molecular weight of a thermodynamic inhibitor, the better the hydrate suppression performance. For example, in glycols MEG has better performance characteristics than diethylene glycol DEG, and methanol MeOH will outperform ethanol EtOH. This tip of the month provides insight into the relative performance of hydrate inhibitors, and discusses the limitations, pros and cons of the various options. In this TOTM we will discuss the general considerations for thermodynamic inhibitor selection. In Part 2, we will review a case study on the difference in performance between MeOH, MEG and DEG for a gathering system inhibition application.
To give insight into the hydrate suppression performance of various inhibitors, we first want to highlight that thermodynamic hydrate inhibitor suppression is a function of the mol percent of the inhibitor in the free water in the system. The hydrate inhibitor performance plots that will be presented in this tip were developed using the Nielsen and Bucklin [1] correlation, and is plotted with the Hammerschmidt [2] correlation, as well as laboratory equilibrium hydrate inhibition data in Figure 1.
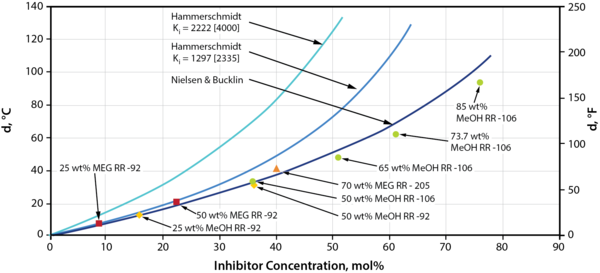
Figure 1. Hydrate Suppression vs. Inhibitor Concentration in Mol %, Comparison and Correlation of Data [3]
Reviewing Figure 1, we see that the Nielsen-Bucklin correlation does an excellent-to reasonable job for all inhibitor concentrations, as compared to the Hammerschmidt equation, in matching the laboratory equilibrium data (data points plotted and referenced in Figure 1).
The Nielsen-Bucklin equation is provided in Equation 1.
(1)
Table 1. provides the molecular weights of the inhibitors that will be used for comparison.
Table 1. Hydrate Inhibitor Molecular Weight (MW)
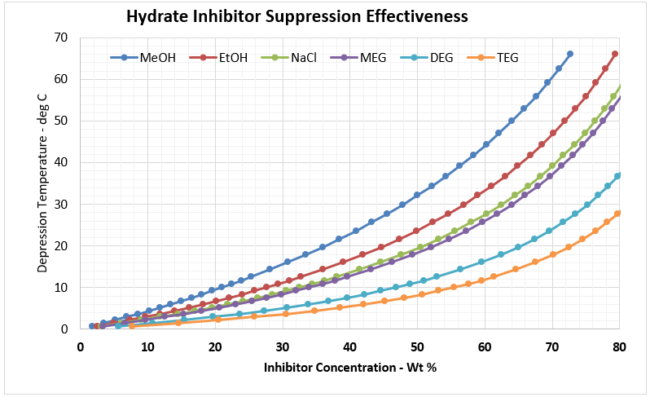
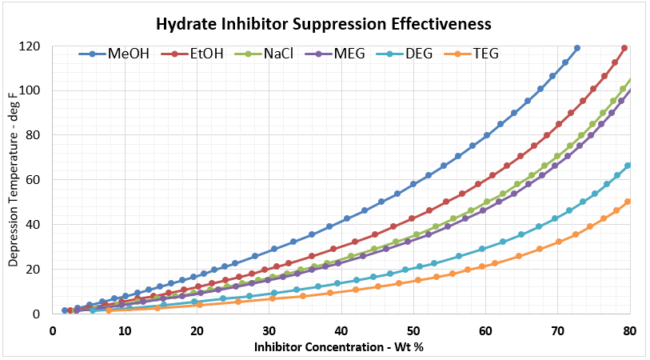
Using Equation 1, and plotting xm as a function of mol %, and then converting the mol% to weight percent (wt%) for each inhibitor results in Figure 2a (SI) and 2b (FPS). Recall, the wt% value is the wt% of the inhibitor required in the free water in the system to prevent hydrate formation.
Figure 2a. Hydrate Inhibitor Suppression Effectiveness
Figure 2b. Hydrate Inhibitor Suppression Effectiveness (FPS)
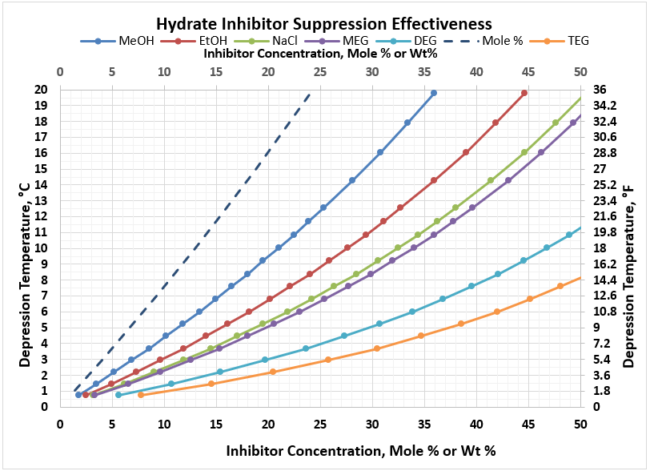
Given these plots are bit too small to read effectively, it is useful to create two separate, more focused plots. In most gathering systems, the depression temperatures required are typically no greater than 20 oC, or 36 oF. Figure 3 provides the useful correlation data that can be used to estimate the required wt% of hydrate inhibitor in the rich water phase for typical pipeline / gathering system conditions.
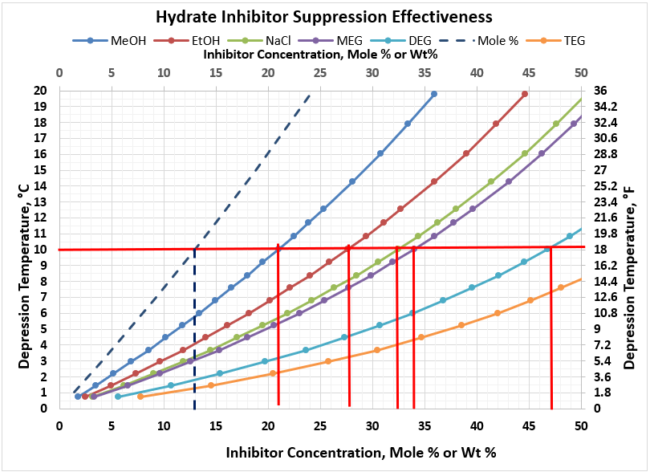
An example of how the various inhibitors compare in terms of suppression performance, let’s assume we needed to achieve a hydrate depression temperature of 10 oC [18oF] to prevent hydrates from forming in our gathering system. Figure 4 provides the wt% of the various inhibitors that would be required for this depression temperature. The results are tabulated in Table 2 below. As shown in Figure 4, the concentration for all inhibitors is the same value of 13 mole %.
Table 2. Comparison of Rich Inhibitor Requirements for 10oC [18oF] Depression T
As can be observed, as the inhibitors MW increases, the amount of inhibitor in the water phase increases for the same depression temperature. Notice TEG is off the chart and would require more than 50 wt% concentration. TEG is not an effective hydrate inhibitor, which is why it is not used in this application. However, it is exceptionally efficient at gas dehydration at very high wt % concentrations, which is why it is the workhorse in the industry in gas dehydration.
Reviewing the remaining inhibitors that can meet this depression temperature requirement, it appears that the oxygenates (MeOH, EtOH) would be the inhibitor of choice as they would require the smallest injection requirements. However, MeOH and EtOH have high vapor pressure, which results in the inhibitor being transported primarily in the vapor phase (which is not where we want the inhibitor to be). As a result of the high vapor pressure, it distributes preferentially into the vapor phase.
Figure 3. Hydrate Inhibitor Suppression Performance for Typical Gathering Systems
Figure 4. Hydrate Inhibitor Suppression Performance for Typical Gathering Systems
In addition, MeOH and EtOH have some mutual solubility with liquid hydrocarbons which is hard to characterize and model. These issues will be discussed further in the Inhibitor Losses and Product Contamination sections.
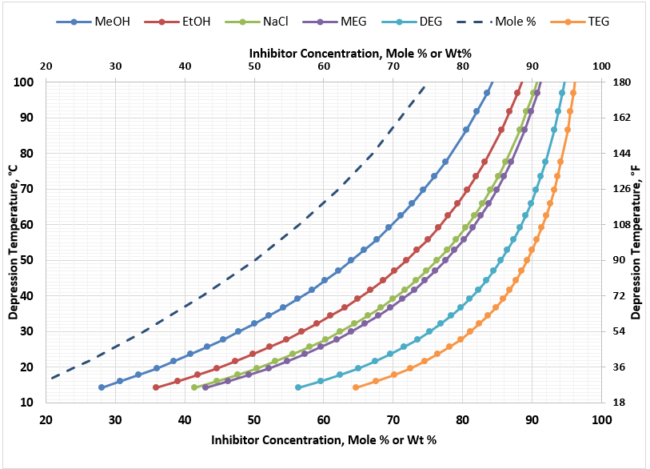
For deep depression temperature requirements, such as that occur in NGL extraction facilities (propane refrigeration or JT valve plants), the range of depression temperatures can be up to 80 oC [144 oF]. Figure 5 provides the hydrate suppression performance of the various thermodynamic inhibitors in this depression range. Again, you see the same characteristics in terms of performance of the inhibitors, however, at these very cold processing temperatures – minimum -40oC [-40oF], the hydrate inhibitor freezing points must be considered. The heavier the glycol becomes, the narrower the window of operation in terms of freezing points becomes. As a result, MEG is the inhibitor of choice for NGL extraction facilities that are operating at cold temperatures.
Figure 5. Hydrate Inhibitor Suppression Performance for Typical NGL Extraction Requirements
Inhibitor Regeneration Requirements
Both MEG and DEG are easily regenerated, whereas MeOH and EtOH present a more difficult regeneration problem as they form an azeotrope with water and aromatics require a complex distillation design. The relative volatility between water and methanol is only 2.5, which indicates a very difficult separation (i.e. very tall and expensive distillation tower). There are only a limited number of installations where this has been done.
When MeOH is used in gathering system hydrate inhibition applications, typically it will not be recovered. The methanol will travel with the various streams, gas, liquid, produced water which ultimately can result in operating problems downstream.
Glycols are nearly always regenerated because of its high purchase cost. Essentially the glycol is heated up in cross exchangers with the regenerated hot lean glycol, and then flows to a reboiler which boils the water concentration out of the glycol to the desired lean concentration. Typical lean glycol concentrations for hydrate inhibition range from 60 – 80 wt %. When MEG used in production systems vs. NGL extraction (refrigeration or JT valve) plants, they will have different regeneration issues.
For NGL extraction facilities, the general issues are emulsions with the liquid hydrocarbons, hydrocarbon carryover to the regenerator, and aromatic emissions (potentially), from the regenerator.
For production systems, the MEG is often times inhibiting produced water which will have some amount of salinity from the reservoir. The rich MEG from the inlet slug catcher of the facility will contain those water with the dissolved salts which will then flow to the regeneration system. In conventional MEG regeneration units, the salt will accumulate and precipitate in the regeneration system causing significant fouling, corrosion and likely result in MEG thermal decomposition [3]. Additional equipment will be required to remove the salts from the MEG. These processes are licensed and are an additional expense that needs to be considered and planned for in the hydrate inhibitor selection process.
Inhibitor Losses / Product Contamination
Despite oxygenates excellent performance in terms of hydrate suppression, their biggest downfall is their distribution behavior. Methanol will distribute in EVERY phase in the system. In fact, the losses are significantly greater than the concentration required in the water phase which results in higher injection rates required as compared to MEG for example
Once you inject methanol into the pipeline, it is in the gas, the condensed water, and the liquid hydrocarbons. This is becoming a more significant issue as of late. Too much MeOH in the produced water can kill the bugs in the produced water treatment system (and it is toxic to fish so produced water offshore could not go overboard). In addition, oxygenates are a petrochemical catalyst poison. Many North American natural gas, y-grade (NGL), and liquid products pipeline tariffs are specifying a maximum oxygenate concentration – or specifying ZERO! In addition, methanol will slowly kill your very expensive 4A molecular sieve dehydration bed over time.
Glycols on the other hand have very low vapor pressure, with minimal losses in the vapor phase. In addition, there is very little mutual solubility with liquid hydrocarbons, so the glycol STAYs where you want it to be, in the water phase.
Inhibitor Cost
Methanol is cheap in comparison to glycols. As a rough rule of thumb, glycols are roughly three times the cost of methanol. That is why glycols are nearly always regenerated. You only need to invest in the chemical costs once. However, methanol is cheap, but you will need to continually buy the amount of chemical you are injecting as it goes downstream and is not recovered.
Summary
This TOTM provided some insight into the various thermodynamic inhibitors’ suppression performance and other considerations. In Part 2 we will review a Case Study in the application and selection of inhibitor for a gathering system inhibition application. In addition, the pro’s and con’s of oxygenates versus glycols will be discussed in more detail.
To learn more about similar cases and how to minimize operational problems, we suggest attending our G4 (Gas Conditioning and Processing) and G5 (Practical Computer Simulation, and Applications in Gas Processing) courses.
By: Kindra Snow-McGregor, P.E. & Mahmood Moshfeghian, Ph.D.
Sign up to receive monthly Tip of the Month emails!
References:
- Nielsen, R.B., and R.W. Bucklin, “Why not Use Methanol for Hydrate Control?”, Hyd. Proc., Vol. 62, No. 4 (Apr. 1983), p. 71.
- Hammerschmidt, E,G., preprint of a paper given at 1939 Tulsa meeting of the Natural Gas Section of the American Gas Association.
- Campbell, J.M., “Gas Conditioning and Processing, Volume 2: The Equipment Modules,” 9thEdition, 3rdPrinting, Editors Hubbard, R. and Snow–McGregor, K., Campbell Petroleum Series, Norman, Oklahoma, PetroSkills 2018.
- Moshfeghian, M.,http://www.jmcampbell.com/tip-of-the-month/2020/03/impact-of-process-gas-pressure-on-the-performance-of-a-mechanical-refrigeration-plant/, PetroSkills -John M. Campbell Tip of the Month, March 2020.
- Moshfeghian, M.,http://www.jmcampbell.com/tip-of-the-month/2019/06/impact-of-temperature-approach-of-the-heat-exchangers-on-the-capex-and-opex-of-a-mechanical-refrigeration-plant-with-meg-injection/, PetroSkills -John M. Campbell Tip of the Month, April 2019
- GCAP 9.3.2, Gas Conditioning and Processing, PetroSkills/Campbell, Tulsa, Oklahoma, 2020.
- Campbell, J.M., “Gas Conditioning and Processing, Volume 1: The Fundamentals,” 9thEdition, 3rdPrinting, Editors Hubbard, R. and Snow–McGregor, K., Campbell Petroleum Series, Norman, Oklahoma, PetroSkills 2018